 Whitebark pine
Whitebark pine
Pinus albicaulis Engelm.
Introduction
Whitebark pine is a medium-sized tree growing in the high mountains. It grows scattered but becomes increasingly abundant towards the south of its range. It is confined to dry, exposed sites at timberline. In British Columbia, it usually grows in even-aged, pure or mixed-species stands. With increasing elevation, the trees grow more scattered and in islands. Whitebark pine is valued for watershed protection and aesthetics (Klinka et al. 2000). Its seeds are fairly large and are an important food for wildlife. Due to the effects of fire suppression, introduced fungal disease (white pine blister rust – Cronartium ribicola) and mountain pine beetle (Dendroctonus ponderosae), population densities of whitebark pine have decreased drastically in the Northern Rocky Mountains (Tomback et al. 1995), and in the southern part of British Columbia local extirpation of the species may be possible.
The range of whitebark pine covers the central and south of the Pacific and central and south of the Cordilleran region (Little 1971). Less than half of the species’ range is inside British Columbia.
Distribution and Protected Areas – from Hamann et.al. 2005
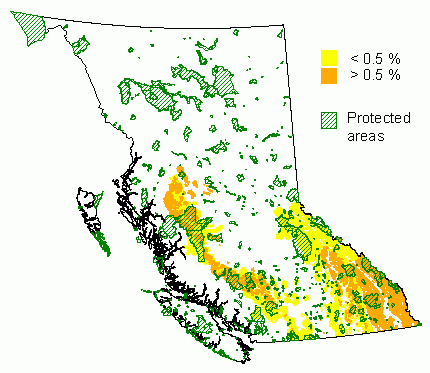
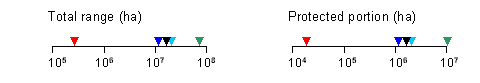
Conservation Status Summary – from Chourmouzis et.al. 2009
No in situ conservation concerns are identified for this species.
Ex situ collections
A small trial plantation of first-generation wind-pollinated seedlings from resistant whitebark pine parents was established at Marks Butte near Clarkia, Idaho, in 1979 (Hoff 1980).
Reproduction
Cone crops are irregular and sometimes large, and seed predation is intense. Germination rates under field conditions are low (8-14%), due to developmental factors (McCaughey and Schmidt 1990). In B.C. the species appears to be well represented in protected areas. However, it is severely at risk in the United States.
Genetic structure
Clark’s nutcrackers (Nucifraga columbiana) play an essential role in whitebark pine reproduction. They hide seeds for winter food, and often, cached seeds are from the same maternal parent. As a result, caches of seeds that germinate result in clumps of closely related trees (Furnier et al. 1987; Rogers et al. 1999). The resulting level of inbreeding is fairly high, but within the range of other bird-dispersed stone and piñon pines (Krakowski 2001). Inbreeding is substantial, resulting primarily from self-pollination but also from some consanguineous mating, with an estimated outcrossing rate of around 70 % (Krakowski 2001). The majority of genetic variability occurs within populations (Jorgensen and Hamrick 1997; Bruederle et al. 1998; Krakowski 2001). Populations in British Columbia are only moderately differentiated (Krakowski 2001). Insights from historical seed movement may provide a basis for recovery plans for this threatened species (Richardson et al. 2002).
Resource management and seed transfer
There is a need for screening for blister rust resistance and for reintroducing wildfires to avoid successional replacement of whitebark pine by fir and spruce. Whitebark pine is extremely susceptible to blister rust (Bingham 1972). Yet, genetically resistant trees have been found (Hoff and Hagle 1990). Four different defense mechanisms were observed in resistant plants (Hoff and Hagle 1990). Many attempts have been made to cross whitebark pine with the other four, more blister rust resistant white pine species in its subsection Cembrae and with most species in subsection Strobi. Almost all have ended either in failure or with inconclusive results (Bingham et al. 1972). Thus, hybridization does not offer much potential for creating more rust-resistant genotypes. No putative hybrids of whitebark pine have been identified in natural stands.
REFERENCES
Hamann, A., Smets, P., Aitken, S. N. and Yanchuk, A. D. 2005. An ecogeographic framework for in situ conservation of forest trees in British Columbia. Can. J. For. Res. 35:2553-2561. View online resources for this report.
C. Chourmouzis, A.D. Yanchuk, A. Hamann, P. Smets, and S.N. Aitken. 2009. Forest Tree Genetic Conservation Status Report 1: In situ conservation status of all indigenous BC species. Centre for Forest Conservation Genetics, Forest Genetics Council of BC, and BC Ministry of Forests and Range, Forest Science Program, Victoria, BC Technical Report 053. www.for.gov.bc.ca/hfd/pubs/Docs/Tr/Tr053.htm
Bingham, R. T. 1972. Taxonomy, crossability, and relative blister rust resistance of 5-needled white pines. Miscellaneous Publication 1221.
Bingham, R. T., Hoff, R. J. and Steinhoff, R. J. 1972. Genetics of western white pine. Res. Pap. WO-12.
Bruederle, L. P., Tomback, D. F., Kelly, K. K. and Hardwick, R. C. 1998. Population genetic structure in a bird-dispersed pine, Pinus albicaulis (Pinaceae). Canadian Journal of Botany 76:83-90.
Furnier, G. R., Knowles, P., Clyde, M. A. and Dancik, B. P. 1987. Effects of avian seed dispersal on the genetic structure of whitebark pine populations. Evolution 41:607-612.
Hoff, R. J. 1980. Unpublished data on file at: USDA Forest Service, Intermountain Forest and Range Experiment Station, Forestry Sciences Laboratory, Moscow, ID.
Hoff, R. J. and Hagle, S. 1990. Diseases of whitebark pine with special emphasis on white pine blister rust. In: W. C. Schmidt and K. J. McDonald (Eds.), Proceedings: symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource, Bozeman, MT, March 29-31, 1989. General technical report INT 270, 386 p.
Jorgensen, S. M. and Hamrick, J. L. 1997. Biogeography and population genetics of whitebark pine, Pinus albicaulis. Canadian Journal of Forest Research 27:1574-1585.
Klinka, K., Worrall, J., Skoda, L. and Varga, P. 2000. The distribution and synopsis of ecological and silvical characteristics of tree species of British Columbia’s forests. Canadian Cartographics Ltd., Vancouver. 180 p.
Krakowski, J. 2001. Conservation genetics of whitebark pine (Pinus albicaulis Engelm.) in British Columbia. MSc thesis thesis. Department of Forest Sciences, Faculty of Forestry University of British Columbia, 107 p.
Little, E., L., Jr. 1971. Atlas of United States trees, volume 1, Conifers and important hardwoods. U.S. Department of Agriculture. 9 p, 313 maps, Washington, DC.
McCaughey, W. and Schmidt, W. 1990. Autecology of whitebark pine (Pinus albicaulis Engelm.). In: W. C. Schmidt and K. J. McDonald (Eds.), Proceedings: symposium on whitebark pine ecosystems : ecology and management of a high-mountain resource, Bozeman, MT, March 29-31, 1989. General technical report INT 270, 386 p.
Richardson, B. A., Brunsfeld, J. and Klopfenstein, N. B. 2002. DNA from bird-dispersed seed and wind-disseminated pollen provides insights into postglacial colonization and population genetic structure of whitebark pine (Pinus albicaulis). Molecular Ecology 11:215-227.
Rogers, D. L., Millar, C. I. and Westfall, R. D. 1999. Fine-scale genetic structure of whitebark pine (Pinus albicaulis): Associations with watershed and growth form. Evolution 53:74-90.
Tomback, D. F., Clary, J. K., Koehler, J., Hoff, R. J. and Arno, S. F. 1995. The effects of blister rust on postfire regeneration of whitebark pine – the Sundance burn of Northern Idaho (USA). Conservation Biology 9:654-664.
