 Limber pine
Limber pine
Pinus flexilis James
Introduction
Limber pine is long-lived, slow-growing, high-elevation tree. Small to medium in size, it often grows on rocky ridges and its form is often irregular. At the lower tree line, it may form either pure stands or mixtures with other conifers. Being relatively shade-intolerant, it is mostly a pioneer species, and maintains its presence only on very severe sites. It provides valuable cover, shelter and nesting opportunities. The large seeds are a nutritious food source for birds, rodents, bears and people. It is useful for reforestation of avalanche paths. It can withstand very windy and dry environments quite well. Its aesthetic value is high (Burns and Honkala 1990; Klinka et al. 2000). White pine blister rust (Cronartium ribicola) is a serious concern for limber pine in Alberta, B.C., and the northern part of Montana. In this area, over 33% of the limber pines are dead and about 75% of the remaining live trees are infected. Further south, the concern gradually lessens, and the disease is largely absent in the southern half of the species’ range (Kendall et al. 1996; Kendall and Schirokauer 1997; Kendall 2001).
Limber pine grows in the Cordilleran, from Alberta and southeastern British Columbia to New Mexico, Arizona, and eastern California. Since it grows at higher altitudes, its distribution is disjunct. Only a small part of the species’ distribution is in B.C. (Little 1971; Burns and Honkala 1990).
Distribution and Protected Areas – from Hamann et.al. 2005
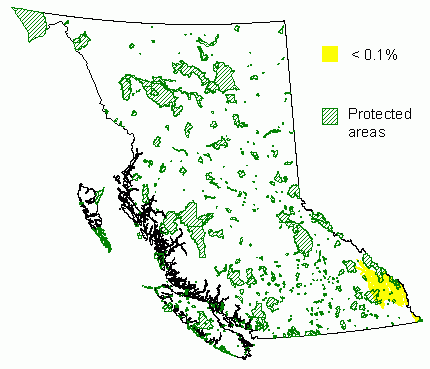
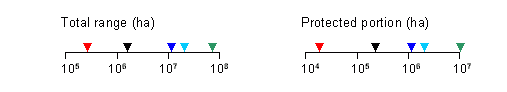
Conservation Status Summary – from Chourmouzis et.al. 2009
“Limber pine has a very limited distribution in British Columbia. The northern edge of its western North American range extends into the southeastern portion of the province, where it is very sparsely distributed in the ESSF, MS, and IDF zones. It is under-protected in all three zones. Field verification is recommended in the IDF zone and in large protected areas that span at least two zones: the IDF/MS zones and the MS/ESSF zones. Considering that there are serious insect and fungal threats to this species, it would be prudent to increase ex situ conservation and implement active management efforts for this species.”
Reproduction
Limber pine produces large seed crops every two to four years. Most of the seeds are wingless and rely on rodents and birds for dispersal. The Clark’s nutcracker (Nucifraga columbiana) caches the seeds in places where little snow accumulates, so their food can be easily accessed. This bird is the most important dispersal agent and may influence the location of limber pine stands more than the pine itself (Burns and Honkala 1990). Trees growing from cached seeds form clusters of related individuals (Carsey and Tomback 1994). The influence of squirrels on seed dispersal was inferred from their influence on the cone and seed traits of limber pine (Benkman 1995).
Genetic structure
Several studies have investigated population structure in limber pine. Schuster and Mitton (2000) studied an isolated population in Colorado using 28 allozyme loci. Analysis of needle tissues provided the following estimates for genetic diversity: the average number of alleles per locus (A) was 2.36; the percentage of polymorphic loci (P) was 50%; the expected number of heterozygotes (He) was 0.159. Latta and Mitton (1997) examined genetic differentiation among seven populations in Colorado, using four types of gene marker: mtDNA, cpDNA, allozymes and RAPDs. Both mtDNA and RAPDs indicated a historical division between populations, corresponding to the continental divide, rather than genetic variation associated with an environmental gradient. Four of the nine RAPD loci were significantly more differentiated than allozymes. This difference could not fully be explained by a historical division, and these four RAPD loci appear to be under natural selection. Mitton et al. (2000a) used maternally-inherited mitochondrial DNA to infer the number of glacial refugia for the species’ range. Population differentiation for this marker was large (FST =0.80). They inferred seven glacial refugia, where either existing haplotypes became fixed or new ones originated via mutation. One surprising result was that of the two populations sampled from Alberta, the northernmost contains a unique haplotype and the other a common one (Mitton et al. 2000a). No B.C. populations were included in the study. Constructing phylogeographies based on mtDNA to distinguish between historical scenarios has been difficult because there are so few mitochondrial markers available. However, seven new primers, developed to amplify the b/c intron of the mitochondrial nad-I gene, allow amplification of smaller fragments than before. These fragments can be distinguished on agarose gels, which will make such projects both cheaper and easier in the future (Mitton et al. 2000b). Several studies have also investigated the amount of gene flow. Schuster et al. (1989) studied 8 populations of limber pine across an elevational transect in Colorado. Most sites differing more than 400 m in elevation did not have overlapping reproductive phenology. Ten isozymes were analyzed for the lowest and highest-elevation population. Allele frequencies differed significantly and both populations had ‘unique’ alleles. Population differentiation, estimated by FST =0.022, was low, indicating a high rate of gene flow. They proposed a stepping-stone pollen transfer model between intermediate populations. The resulting estimates for gene flow via pollen correspond to those estimated by paternity analysis (Schuster and Mitton 2000), which estimated mean pollen dispersal distance at 140 m. Latta and Mitton (1997) found that, for seven populations in Colorado, maternally inherited chloroplast DNA was much more differentiated than paternally inherited mitochondrial DNA, indicating that gene flow via pollen is much larger than gene flow via seeds.
Resource management and seed transfer
The primary challenge for genetic resource management of limber pine is white pine blister rust in the northern portion of the range. Rust resistance has been found and bred for in western white pine (P. monticola). A possible management option for limber pine would be to introducing resistance through hybridizing with these rust-resistant western white pine individuals. However, crossability barriers between the species (e.g., Kriebel 1972; Quijada et al. 1997; Bingham 1972) remain a problem. Introducing an exotic genome would also present more risks than identifying resistant individuals within the native species. The latter approach seems preferable, but no efforts have been made to date, likely because of the limited commercial importance of the species. Schoettle and Rochelle (2000) found similar growth for limber pines growing over a range of elevations (1600 to 3300 m), indicating plasticity with regard to the factors changing with elevation. Such plasticity would facilitate the deployment of any rust resistant trees in the field.
REFERENCES
Hamann, A., Smets, P., Aitken, S. N. and Yanchuk, A. D. 2005. An ecogeographic framework for in situ conservation of forest trees in British Columbia. Can. J. For. Res. 35:2553-2561. View online resources for this report.
C. Chourmouzis, A.D. Yanchuk, A. Hamann, P. Smets, and S.N. Aitken. 2009. Forest Tree Genetic Conservation Status Report 1: In situ conservation status of all indigenous BC species. Centre for Forest Conservation Genetics, Forest Genetics Council of BC, and BC Ministry of Forests and Range, Forest Science Program, Victoria, BC Technical Report 053. www.for.gov.bc.ca/hfd/pubs/Docs/Tr/Tr053.htm
Benkman, C. W. 1995. The impact of tree squirrels (Tamiasciurus) on limber pine seed dispersal adaptations. Evolution 49, 585-592.
Bingham, R. T. 1972. Taxonomy, crossability, and relative blister rust resistance of 5-needled white pines. Miscellaneous Publication 1221. U.S. Department of Agriculture. Washington, DC. 271-280.
Burns, R. M. and Honkala, B. H. 1990. Silvics of North America. Agriculture handbook 654. U.S. Dept. of Agriculture Forest Service, Washington, D.C. 877 p.
Carsey, K. S. and Tomback, D. F. 1994. Growth form distribution and genetic relationships in tree clusters of Pinus flexilis, a bird-dispersed pine. Oecologia 98, 402-411.
Kendall, K. C., Ayers, D. and Schirokauer, D. 1996. Limber pine status from Alberta to Wyoming. Nutcracker Notes 7:23-24. USDA Forest Service, Intermountain Research Station, IFSL. Missoula, MT.
Kendall, K. C. and Schirokauer, D. 1997. Alien threats and restoration dilemmas in whitebark and limber pine communities. p. 218-225 in: Proceedings of the George Wright Society Conference on Research and Resource Management in Parks and on Public Lands Vol. 9.
Kendall, K. C. 2001. Limber pine communities. U.S. Department of the Interior, U.S. Geological Survey, Northern Rocky Mountain Science Center, Montana State University (October 2002). [Online]. Available: http://www.nrmsc.usgs.gov/research/limber.htm [Last Modified: 2 November 2001]
Klinka, K., Worrall, J., Skoda, L. and Varga, P. 2000. The distribution and synopsis of ecological and silvical characteristics of tree species of British Columbia’s forests. Canadian Cartographics Ltd., Vancouver. 180 p.
Kriebel, H. B. 1972. Embryo development and hybridity barriers in the white pines (Section Strobus). Silvae Genetica 21:39-44.
Latta, R. G. and Mitton, J. B. 1997. A comparison of population differentiation across four classes of gene marker in limber pine (Pinus flexilis James). Genetics 146: 1153-1163.
Little, E., L., Jr. 1971. Atlas of United States trees, volume 1, Conifers and important hardwoods. U.S. Department of Agriculture. 9 p, 313 maps, Washington, DC.
Mitton, J. B., Kreiser, B. R. and Latta, R. G. 2000a. Glacial refugia of limber pine (Pinus flexilis James) inferred from the population structure of mitochondrial DNA. Molecular Ecology 9:91-97.
Mitton, J. B., Kreiser, B. R. and Rehfeldt, G. E. 2000b. Primers designed to amplify a mitochondrial nad1 intron in ponderosa pine, Pinus ponderosa, limber pine, P. flexilis, and Scots pine, P. sylvestris. Theoretical & Applied Genetics 101:1269-1272.
Quijada, A., Liston, A., Robinson, W. and Alvarez-Buylla, E. 1997. The ribosomal ITS region as a marker to detect hybridization in pines. Molecular Ecology 6:995-996.
Schoettle, A. W. and Rochelle, S. G. 2000. Morphological variation of Pinus flexilis (Pinaceae), a bird-dispersed pine, across a range of elevations. American Journal of Botany 87:1797-1806.
Schuster, W. S., Alles, D. L. and Mitton, J. B. 1989. Gene flow in limber pine: evidence from pollination phenology and genetic differentiation along an elevational transect. American Journal of Botany 76:1395-1403.
Schuster, W. S. F. and Mitton, J. B. 2000. Paternity and gene dispersal in limber pine (Pinus flexilis James). Heredity 84:348-361.
